Covalent compounds Ionic compounds composed of simple molecules a Have high melting and boiling points a Have low melting and boiling points b Exist as solids at room temperature. Only two elements are liquid at.

Inorganic Nomenclature Flow Chart Chemistry Education Teaching Chemistry Chemistry Lessons
The correct answer is option A.

. H2 E CH The apparatus pictured below is used to conduct the following experiment. B covalent compounds are all gases but ionic compounds may be solids liquids or gases. 10 which two properties are more typical of molecular compounds than of ionic compounds.
While the ions in an ionic compound are strongly attracted to each other covalent bonds create molecules that can separate from each other when a lower amount of energy is added to them. Most covalent compounds have relatively low melting points and boiling points. For example molecular compounds that have a very low molar mass like ceCO and ceCH4.
Therefore molecular compounds usually have. They are mercury a metal and bromine a halogen. The reason these elements are liquids has to do with how tightly bound their electrons.
They have high mehing points 3. Only two elements on the periodic table are elements at room temperature. 4 and 2 D.
At room temperature simple molecular substances are gases or liquids or solids with low melting and boiling points. A 100 atm 760 torr B 152 mm Hg 203 x 104. Atoms share electrons a 1 and 4b1 and 3 2 and 3 2 and 4 3 and 4 -11.
Previous question Next question. Which of the following is most likely not a gas at room temperature. Solids do not conduct electricity but liquids do 4.
And the neat reagent is treated with great respect. These compounds are found in all three physical states at room temperature. OTHER SETS BY THIS CREATOR.
Volatile c Conduct electricity in the molten state or in an aqueous solution but do not conduct electricity. Answer 1 of 6. A Cl2 B CH4 C H2 D HCl E Licl 23 Which of the following equations shows an incorrect relationship between pressures given in.
Both of theses molecules have a very small molar mass a very low London dispersion force and thus a very low boiling point. In fact nearly all room-temperature liquids are molecular compounds. Textdibromine is a room temperature LIQUID as Br_2 and it is one of the nastiest corrosive things you can handle in the laboratory.
Four other elements are liquids slightly warmer than room temperature. Molecular compounds of low molecular weight tend to be gases at room temperature Which of the following is most likely not a gas at room temperature RbCI HCI CH HCN Cl2. A single crystalline nanowire of an organic room-temperature-phosphorescent compound E-3-4-nitrophenyliminomethyl-2H-thiochroman-4-olateBF 2 S-BF2 was prepared by self-assembly in.
List three specific tests that you could perform to figure out whether they are ionic or molecular. 22 Molecular compounds of low molecular weight tend to be gases at room temperature. Compounds that are gases at room temperature are all covalent compounds such as CO 2 SO 2 and NH 3 that contain two or more.
Start studying Compounds that are liquids at room temperature. Which of the following is most likely not a gas at room temperature. Simple molecular substances generally have low melting points and boiling points and are often liquids or gases at room temperature.
They are gases or liqaids at room temperature. View the full answer. This is because RbCl has the highest molecular weig.
Dinitrogen and carbon dioxide and argon are ALL room temperatu. In general at room temperature a ionic compounds are all solids but covalent compounds may be solids liquids or gases. Molecular compounds of low molecular weight tend to be gases at room temperature.
Elements that are gases at room temperature are all nonmetals such as He Ar N 2 O 2 and so on. They are not brittle 5. While covalent bonds between atoms are quite strong attractions between moleculescompounds or intermolecular forces are relatively weak.
Properties of Covalent Compounds. Learn vocabulary terms and more with flashcards games and other study tools. Common gases at room temperature include both elements such as H 2 and O 2 and compounds such as CO 2 and NH 3.
Non-volatile b Usually exist as liquids or gases at room temperature. 3 and 5 E 3 and 4 11. Chemistry questions and answers.
Naming Covalent Compounds Set 2. They do not conduct electricity. 5 and 2 C.
You have been given four substances that are all white solids at room temperature. The crystal structures Supplementary Information S4 of Phz-H 2 xa have nonpolar space group P2 1 n at room temperature and the b axis with the shortest lattice constant is parallel to the. Molecular Formulas with subscripts.
The most common example is water a molcular compound that is liquid at room temperature. Which two properties are more typical of molecular compounds than of ionic compounds. Delta G_textfusionDelta H_textfusion-TDelta S_textfusion where Delta H.
Fusion at room temperature. They have high melting points. They are francium cesium gallium and rubidium all metals.
1 and 4 B. Covalent compounds usually have lower enthalpies of fusion and vaporisation than ionic compounds. Ionic compounds have a high melting point while molecular compounds have a low melting pointIf I have a white solid this solid may be ionic or molecular.
What is the molar mass of toluene if 085 g of. After complete evacuation of both chambers valve b is closed and a sample of CO-g is introduced through. They are solids at room temperature.

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts

Afbeelding Overzicht En Vergelijking Van Eigenschappen Van Ionische En Covalente Verbindingen Teaching Chemistry Chemistry Classroom Chemistry Lessons
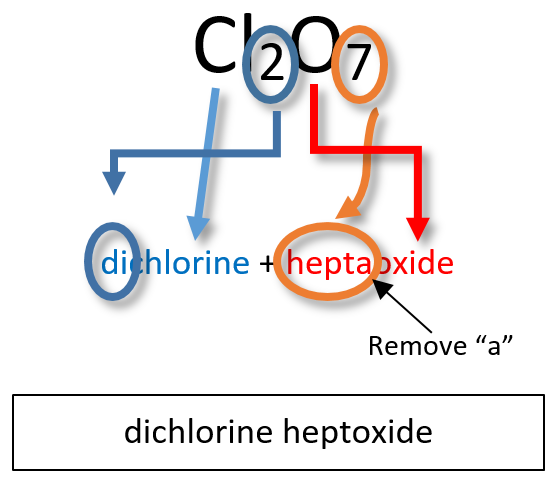
Ch103 Chapter 5 Covalent Bonds And Introduction To Organic Molecules Chemistry

Covalent Compound Properties Molecular Geometry Higher Order Thinking Skills Higher Order Thinking

Penguinone Is An Organic Compound With The Molecular Formula C10h14o Its Name Comes From Th Cooking Steak On Grill How To Cook Steak Cooking With Coconut Oil

Ionic Vs Molecular Compounds Final Gcse Chemistry Chemistry Help Secondary Science
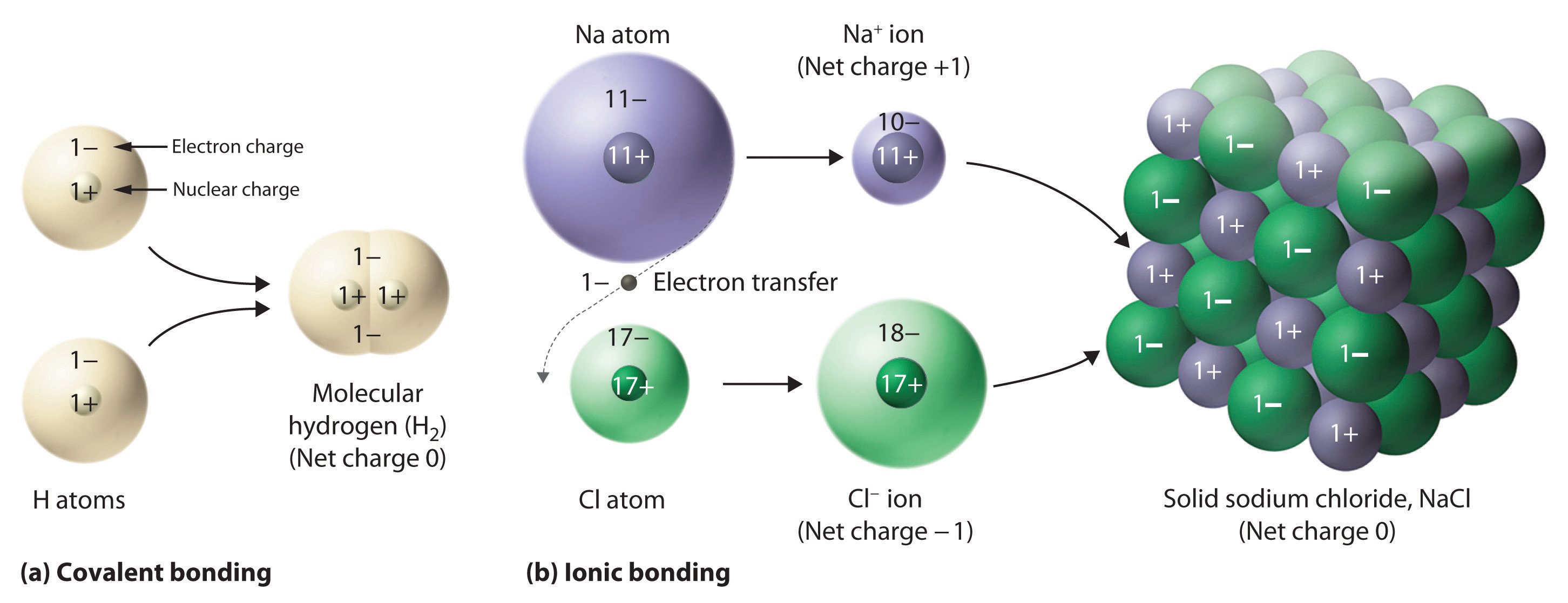
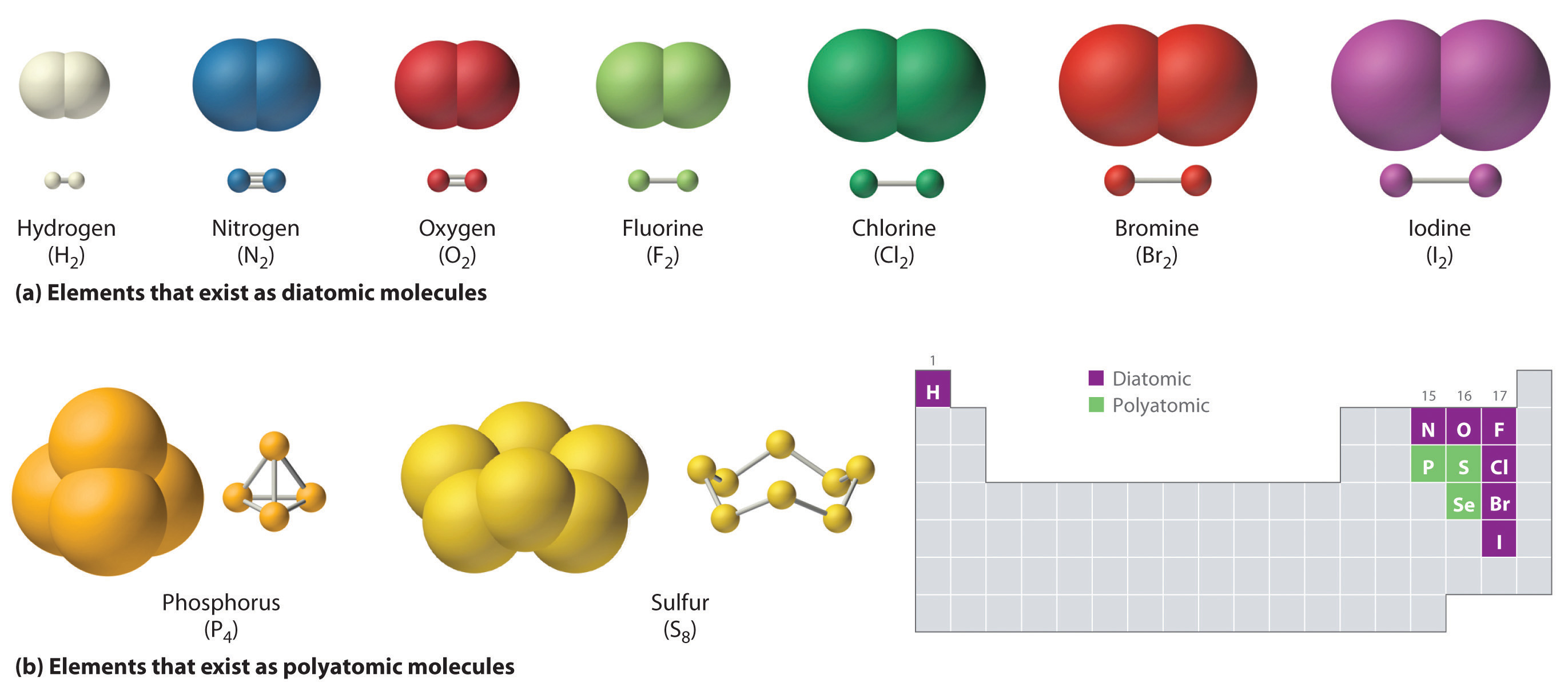
0 comments
Post a Comment